With pressures to drive down costs, remain compliant, and improve innovation and the safety of products, manufacturers across all industries are increasing their focus on quality management. Executives are relying on a variety of resources — across people, processes, and technology — to progress forward and achieve process excellence. But before a company begins an initiative related to improving quality maturity, it’s important to understand the various phases of maturity and to define objectives for how to advance maturity from one phase to the next.
Ahead of the American Medical Device Summit, we spoke with Roxane Napoli, Associate Director of Product Marketing at IQVIA Quality Compliance, to discuss how organizations within the medical device space can progress forward and improve their quality management capabilities.
Below, we cover:
- IQVIA’s quality maturity model for life sciences
- How increasing levels of quality maturity will benefit the business
- The components of a balanced quality maturity initiative (People, Processes, and Technology)
- How technology can improve quality maturity efforts
- The relationship between quality maturity and improved decision making and why this is critical to the success of the quality program
- How organizations that are functioning at high maturity levels can continue to maintain and improve their leadership position
What is IQVIA’s quality maturity model for life sciences?
We approach quality challenges from a maturity perspective. At any given time, a company’s maturity level falls within a range that scales from development (low-quality maturity) to leadership (high maturity). In our framework, quality maturity is driven by people and processes, and fueled by technology. Our goal is to help our customers get to higher stages of maturity to gain the benefits of higher product quality, improved compliance, and reductions in overall cost of quality.
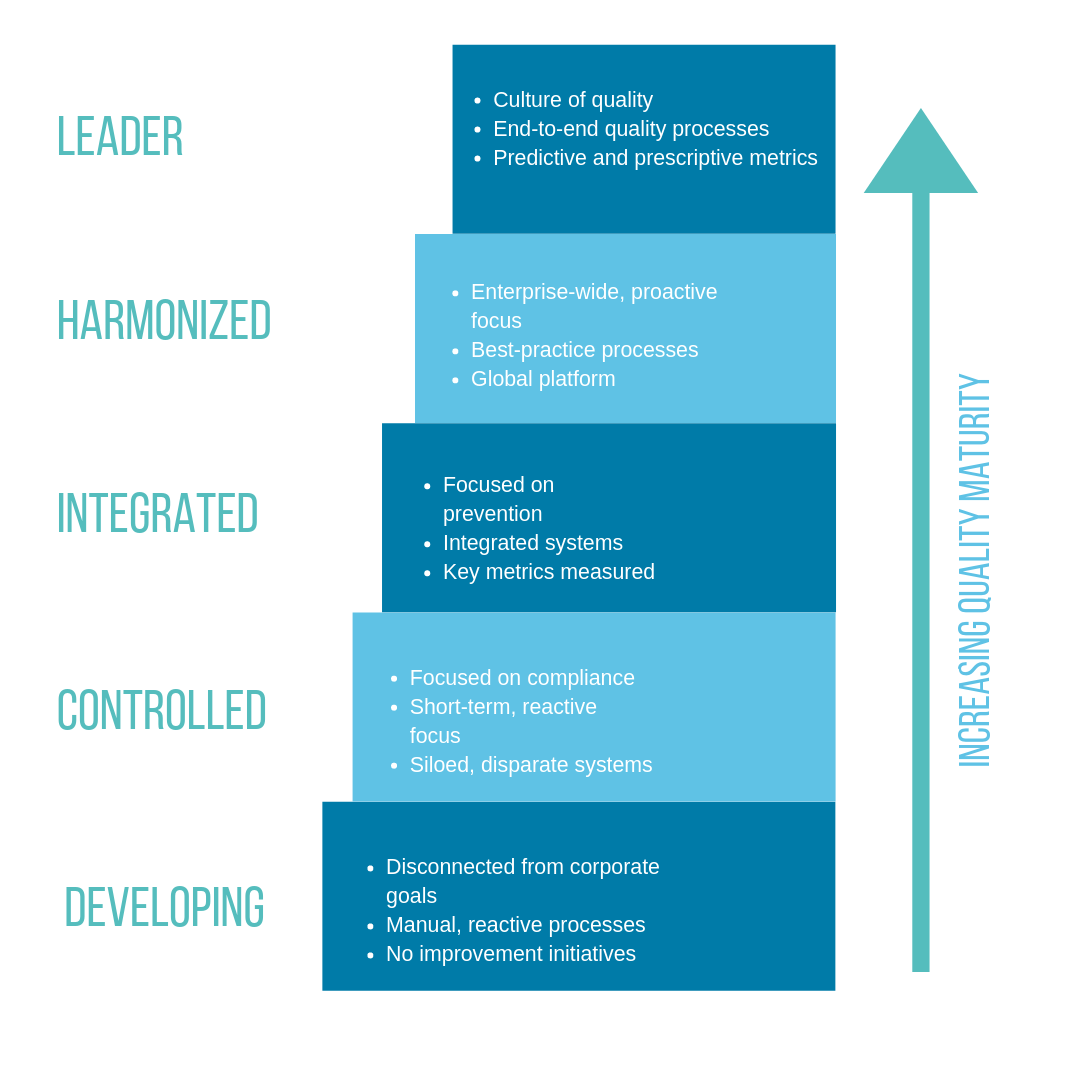
In the beginning stages of maturity, processes are manual and reactive. They’re mainly compliance-driven as opposed to driven by quality improvement. Many organizations in this stage are facing the challenges of GMP readiness and compliance with new and evolving regulations, such as EU MDR for the medical device industry. At this phase of maturity, quality is seen more as a function or department. Many businesses at this phase are using a variety of point technology solutions that aren’t integrated with one another, or they are still using paper-based processes. These contribute to an overall lack of visibility and accountability for quality processes and results.
As organizations increase in maturity, the focus moves to prevention and the organization becomes better able to anticipate and manage risk. This can be driven and supported by integrated quality and IT systems.
In higher stages of quality maturity, companies become more proactive and can harmonize processes across the business and even beyond their four walls to better manage supplier quality and activities. At the highest level of maturity, organizations begin developing a true culture of quality with end-to-end quality processes and the ability for metrics to help sustain and improve overall quality.
It’s also possible to move back and forth between quality maturity levels. It takes ongoing management commitment and a continuous improvement effort to drive maturity forward and to sustain it.
How does increasing levels of quality maturity benefit the business?
As organizations advance on the quality journey, they can more effectively address regulatory challenges, better manage risk, and bring quality systems into alignment.
One of the key impacts that we see is the impact to overall cost of quality. In our customer interactions, we’ve seen a significant decrease in overall Cost of Quality by advancing even one level in quality maturity. For example, moving from Level 2 (Controlled) to Level 3 (Integrated) can reduce overall Cost of Quality by 6-7%. The figure below provides additional details on cost of quality metrics for various levels of quality maturity.

What are the components of a balanced quality maturity initiative?
Quality maturity is developed over time. Organizations that wish to develop or advance their quality maturity would be best served by viewing it as an initiative that requires people, processes, and technology in order to be successful.
There is no “one size fits all” approach to advancing an organization’s quality maturity but there are some steps you can take to get started. The first step for any improvement initiative is understanding the organization’s current state. First, identify where your company sits on the quality maturity scale using the sample provided in Figure 1. In general, your organization is only as mature as your weakest point. For example, you may be focused on prevention (a Level 3 – Integrated characteristic) but utilizing siloed, disparate systems (a Level 2 – Controlled characteristic). If that’s the case, your overall quality maturity is at Level 2. That’s your starting point.
Then, based on the current state, short-term and long-term goals to either advance in maturity level or sustain the current maturity level can be developed. Some of these goals may need to be people-based or process-based, and others will be driven by technology.
How does technology improve quality maturity efforts?
We think of technology as the fuel for advancing quality maturity. In some cases, technology is the driver for moving from one level to the next. For example, it’s difficult to integrate systems, measure key metrics, or have a global platform for quality without a solid technology framework as a foundation. Technology also improves efficiency and can take some of the time-intensive work out of managing a quality system. This, in turn, frees up time and resources and better enables quality compliance personnel to focus more on prevention or other aspirational quality goals, rather than reacting to what’s in front of them now.
Finally, quality technology enables improved decision making, which is critical to advancing maturity. Integrated quality systems with business intelligence capabilities enable the development of quality metrics that drive maximum impact. For example, this can enable the understanding of the most frequently encountered failure mode to be corrected, the isolation of high-risk issues for immediate action, a deeper examination of quality process cycle times, and more. Quality team members can make faster, better decisions since they can quickly identify areas of concern and hone in on them for maximum impact.
How can organizations that function at high maturity levels continue to maintain and improve their leadership position?
Some level of effort is needed to sustain quality leadership over time. We have many organizations that come to us that are already functioning at high maturity levels. Many of them need ongoing support to sustain or improve their maturity initiative. Since high levels of maturity are often tied to technology, many of these organizations need ongoing support and Quality Management System (QMS) enhancements. They may need to consider upgrading their current system, moving to a more robust quality system, or making improvements in their analytics capabilities.
Quality efforts are also closely tied to regulatory and GMP compliance, so it’s important to keep a pulse on upcoming regulations to be sure that they’re being addressed in a proactive manner. Technology upgrades alone will not always be enough to maintain compliance with new regulations. Even when technology can assist with compliance, there are often people- or process-related changes that must occur to stay fully up to speed.
There are also situations where organizations have mature or compliant quality systems that are not necessarily efficient. Looking at improved processes and technology can improve even the most mature quality systems.
How can IQVIA assist companies looking to advance their quality maturity?
IQVIA can assist in advancing your quality maturity, no matter where your organization is right now. By viewing quality as a people, process and technology journey, rather than just a challenge that technology alone can solve, we’re able to provide end-to-end offerings to develop quality leadership within an organization.
Our innovative approach to the market and our tailored Quality Compliance solutions are made possible by the IQVIA CORE™: the integration of unrivaled technology and analytics, market-leading data, and deep domain expertise. This means we can develop solutions specifically tailored for our customers, that ensure they can meet the market’s ever-changing demands. Our life sciences domain expertise makes us more than just a technology supplier – we can put our technology solutions in the context of advancing your quality maturity and solving your business needs, complemented by professional consulting services.
IQVIA provides technology solutions that reach across the whole of the lifecycle, from safety, regulatory, and compliance solutions; through to information, content, and performance management; to multichannel engagement with payers, physicians, and patients; all underpinned by cutting-edge technology that leverages AI and machine learning.
IQVIA Quality Compliance provides the people, processes, and technology to fuel quality maturity. Learn more about our offerings here.
%20(1).png?width=773&height=112&name=Generis%20Logo%20full%20Colour%20(Large)%20(1).png)

.png)
-2.png)
